Chlorophyll
Fluorescence in vivo:
A Theory (Part I)
Most part of the photosynthetic pigments in
phytoplankton cell reside in peripheral pigment-protein complexes of the
light-harvesting antenna (I, see Fig. 1). Absorption of light quantum induces
the transition of the pigment molecule into excited state. From peripheral
antenna complexes, excitation is efficiently transferred to core antenna
complexes near photosynthetic reaction centers (II, Fig. 1), where it can be
used in the primary photochemical reaction of photosynthesis. But a small
fraction of excitons is reemitted as fluorescence or thermally dissipated while
they migrate in the core antenna complex to the reaction center (Fig. 1).
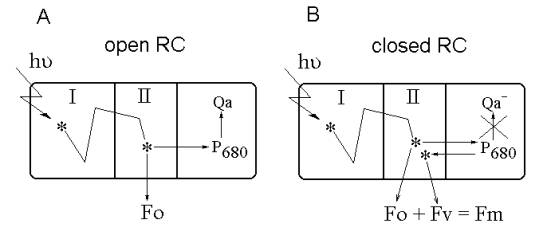
Fig.1. The constant and variable fluorescence origin mechanism.
The fate of exciton is determined by the relative
values of the rate constants of three
concurrent deactivation processes in the core complex:
P* ![]() P
(1)
P
(1)
where:
P and P* - the ground and excited states of
chlorophyll ‘a’ molecule;
kf, kd and kph
- the rate constants of the radiative (fluorescence), nonradiative (thermal
dissipation), and photochemical (phothosynthetical) deactivation of excitons.
The quantum yields of the primary
photosynthetic reaction and fluorescence are equal, respectively, to
fZ = kph
/(kf + kd + kph) and fFo= kf /(kf + kd +
kph) (2)
The rate constant values are dependent on the
molecular organization of the photosynthetic reaction centers and, probably, do
not change with taxonomic composition of phytoplankton. Under optimal
conditions and with active reaction centers, kph is the greatest
from these three constants. As a result, the quantum yield of the excitation
energy use (fZ) is near to unit, and only a small part of the
excitons (about 0.03%) is lost in the form of fluorescence during exiton
migration to the reaction centers.
The
relation between constant fluorescence (Fo)
and
phytoplankton concentration
Measuring the fluorescence yirld with open
reaction centers (Fo) is a fairly simple and convenient method for
estimating phytoplankton concentration. The fluorescence sensors in PrimProd
fluorometer are commonly calibrated in chlorophyll ‘a’ concentration units,
because the fluorescence intensity fairly good correlates with this
phytoplankton concentration parameter (Yentsch and Menzel, 1963), especially for individual algae
species (Fig. 2).
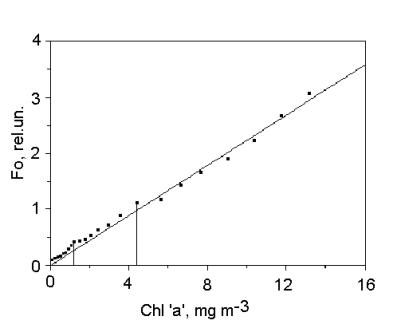
Fig.2. Fo vs. chlorophyll ‘a’ concentration in Chlorella sp.
suspension.
Data were obtained using PrimProd fluorometer.
But such correlation may be low for natural
phytoplankton communities, because the pigment composition of the pripheral
light-harvesting antenna complexes is variable in different algae taxons.
Besides, the ratio of pigments in light-harvesting complexes varies in response
to ambient light intensity, nutrition supply etc. As a result, the
proportion of chlorophyll in bulk photosynthetic pigments changes in a wide
range depending on taxonomic composition and physiological condition of the
phytoplankton studied.
Despite the fact that only chlorophyll
‘a’ is the fluorescence emitter, all the light-harvesting pigments, including
the pigments of the peripheral complexes, supply excitons for the fluorescence.
Fluorescence intensity from a water
sample is given by the equation:
Fo = G*NRC*fFo*Ifl*![]() ifl (l)*S(l)dl
(3)
ifl (l)*S(l)dl
(3)
where:
G = const - a factor determined by the geometry
and sensitivity of the fluorescence sensor;
NRC - the concentration of the photosynthetic reaction centers in a unit
volume;
fFo - the fluorescence quantum yield with reaction centers being in the open
state;
Ifl=![]() Ifl(l)dl - the total measuring (probe) light intensity integrated over the
spectral range, where Ifl(l) -
spectral distribution of the light intensity;
Ifl(l)dl - the total measuring (probe) light intensity integrated over the
spectral range, where Ifl(l) -
spectral distribution of the light intensity;
ifl(l) = Ifl(l)/Ifl
- normalized spectral distribution of the probe light;
S(l)- the absorption spectrom of all the pigments supplying excitons to
reaction centers (i. e. the dependence
of the absorption cross section of the light-harvesting antenna on the exciting
light wavelength).
Considaring that G*Ifl is constant for available fluorometer one can write:
Fo = Q * fFo * NRC
* S (4)
where S = ![]() ifl(l)*S(l)dl - the absorption cross section of the light-harvesting antenna of single
reaction center for given spectral distribution of the exciting light ifl(l);
ifl(l)*S(l)dl - the absorption cross section of the light-harvesting antenna of single
reaction center for given spectral distribution of the exciting light ifl(l);
Q is constant value.
If the light intensity is uniformly distributed
in a spectral range (ifl(l) =
const) and assuming fFo as constant, then S is an integral of the
absorption spectrum of the light-harvesting antenna and Fo is a linear
function of the product NRC*S,
i. e. the total absorptivity of all the reaction centers present in the water sample,
thus Fo could be characteristic of light absorbtion capacity of
phytoplankton (so called aPSP [details in
Part II]).
The value Fo reflects light
absorption by the given phytoplankton community and can be a more adequate
index of phytoplankton concentration than chlorophyll concentration.
In the present model of the fluorometer, the
probe flash is given from a xenon lamp through a blue-green absorption filter
SZS-22. This combination provides nearly uniform spectral distribution of the
exciting light in the range from 380 to 540 nm. Fig. 2 shows, that in chrysophytum (N. salina) and
diatomea (Th. weissflogii) algae, in
which chlorophyll comprises small part of the light-harvesting antenna, Fo
intensity per unit of chlorophyll ‘a’ is about three times higher than in green
alga, which has chlorophyll ‘a’ as a major light-harvesting pigment. These
relation correlates with the pigment index, which reflects carotenoid
contribution in the light absorption.
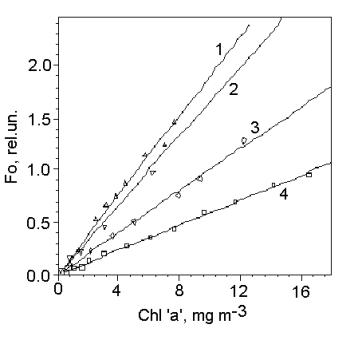
Fig.
3. The dependences of Fo intensity on chlorophyll ‘a’ concentration for
four marine species relating to different algae taxons: diatomea - Thalasiosera weissflogii (1); chrysophyte - Nephrochloris
salina (2) and green algae - Ankistrodesmus sp. (3) and
Platimonas viridis (4). The data were obtained using a
PrimProd fluorometer.
The
variable chlorophyll fluorescence and
the
photosynthetical activity
The light energy conversion in the reaction center
takes some time to be completed. During this time (turnover time), the reaction
center is in so called closed state and can not process a next exciton. In this
state, the rate constant of the photochemical exiton quenching is equal to zero
and the quantum yield of the chlorophyll fluorescence reaches its maximum level
(Fm):
fZ = 0
fFm = kf /( kf + kd )
(5)
The difference between fluorescence intensities
in closed and open reaction centers (Fv=Fm-Fo) is known as the
variable chlorophyll fluorescence; it corresponds to that part of the absorbed light
energy, which would be used in photosynthesis if the reaction centers were in
the open state. It follows from (2) and (5), that the ratio of the variable to
maximum fluorescence yield is equal to the quantum efficiency of the primary
charge separation in photosynthetic reaction centers:
(fFm - fFo)/fFm = kph /( kf + kd +kph)=qZ
(6)
Therefore, measuring the fluorescence
intensities Fo and Fm enables to estimate the efficiency of the
photochemical conversion of absorbed light energy in reaction centers of PS II:
fZ =Fv/Fm
(7)
Relation Fv/Fm can be used as
characteristic of photosynthetical activity of phytoplankton.